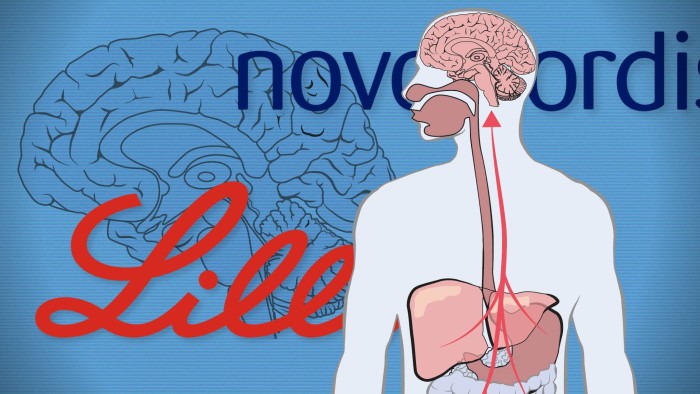
One of Dr Mo Sarhan’s patients was experiencing intense cravings for opioids and alcohol when the Florida-based doctor offered him a striking solution: the Eli Lilly weight-loss drug Mounjaro.
“Within days, all of his cravings were gone and he was much more effective in his engagement and treatment. He’s done great since,” Sarhan says.
Sarhan and his colleague Steven Klein at the Caron Treatment Centers in Florida and Pennsylvania have prescribed a range of so-called glucagon-like peptide-1 receptor agonists (GLP-1s) to treat addictions, using them alongside traditional therapies, to around 75 patients.
The results, which Sarhan describes as “compelling”, are the latest sign that drugs such as Mounjaro, Ozempic and Wegovy could be effective treatments for a startlingly wide range of conditions well beyond their original focus on obesity and diabetes.
On Tuesday, Dave Ricks, Eli Lilly’s chief executive, said the company would begin large studies of GLP-1s in alcohol, nicotine and drug abuse — the first pharmaceutical group to do so.
“These medicines, we think and we’ve aimed to prove, can be useful for other things we don’t think about connected to weight,” he told the Economic Club of Washington DC.
While GLP-1s are not clinically approved for addiction treatment, preliminary studies suggest they reduce cravings by acting on pleasure pathways in the brain — similar to the mechanism that dampens appetite.
In recent years, GLP-1s have helped transform the waistlines of patients and the top lines of pharmaceutical groups Novo Nordisk and Eli Lilly. Worldwide, more than 1bn people are defined as obese, according to research published this year in The Lancet, with rates doubling for adults and quadrupling for children and adolescents between 1990 and 2022. Conditions linked to obesity, such as chronic kidney disease and diabetes, are increasingly prevalent too.
According to data provider Airfinity, there are 66 ongoing, late-stage trials of GLP-1 drugs for obesity, diabetes and a range of other conditions linked to excess weight. The prospect of broader uses for the drugs is one reason that Goldman Sachs analysts anticipate a $130bn market for them by 2030.
But there are also ongoing studies in treatment areas with few apparent links to excess weight. Novo Nordisk is running three late-stage trials of semaglutide, the active ingredient in its Ozempic and Wegovy drugs, for Alzheimer’s disease. Another of the class of drugs, lixisenatide, has shown some early promise in slowing the worsening of motor symptoms in Parkinson’s disease patients.
Widespread uptake of the drugs could help tackle the rising tide of chronic diseases across the world and lower the costs associated with them for health systems.
In one example, Airfinity estimates that Wegovy could prevent up to 3.8mn cases of obstructive sleep apnoea, a breathing disorder, in the US by 2030. That could cut expenditure on the CPAP (continuous positive airway pressure) machines used to help patients manage symptoms in the US by up to $3bn.
Sarhan’s patients, most of whom pay for the drug themselves and are all informed beforehand of its regulatory status, have taken the treatments for addiction to nicotine, inhalants and alcohol. But the therapeutic mechanisms of the drugs are not yet fully understood and many of the benefits have not been verified in late-stage trials.
Production of GLP-1s is currently dominated by Novo Nordisk and Eli Lilly, which have much more incentive to maximise the revenue potential of the obesity market than to spend on experimental clinical trials in unproven treatment areas.
And the hefty price tags and unpleasant side effects associated with current weight-loss treatments mean they are not yet a panacea for the growing body of chronic health conditions across the world.
Lower-priced, more widely available drugs that could be taken orally are “the direction of travel,” says Naveed Sattar, professor at the University of Glasgow and chair of the UK government’s obesity mission. “But we are not there yet — because the drugs are too expensive.”
GLP-1 receptor agonists have surprised scientists before. The gut hormone was originally discovered in the 1980s, but found to break down quickly in the body. It was not until researchers discovered a similar but more stable compound in the venom of the Gila monster, a lizard native to North America, that they were able to produce long-lasting drugs mimicking GLP-1’s effects. The first GLP-1 based drug was approved in 2005.
Early GLP-1s were developed as diabetes treatments owing to their ability to stimulate insulin production, with weight loss initially a useful side effect. Previous treatments for obesity itself came with dangerous side effects. The “fen-phen” cocktail of an appetite suppressant and amphetamine was withdrawn in 1997 after its use was linked to heart valve defects.

Rather than increasing cardiovascular risk, GLP-1s have shown impressive benefits. Last year, Novo Nordisk unveiled data that suggested semaglutide cut the risk of heart attacks by 28 per cent in its so-called Step trial of 17,604 patients, and reduced the risk of death by 18 per cent.
The results of the trial have helped expand the drugs’ usage. In March, Novo Nordisk secured US approval for Wegovy to reduce the risk of heart attacks and strokes in overweight or obese adults with cardiovascular disease.
“It’s a complete turnaround from what we’ve had before,” says Vlado Perkovic, a renal specialist at UNSW Sydney, who has done research on the impact of GLP-1s on kidney conditions. Trials show the drugs are effective in treating both chronic kidney disease and metabolic dysfunction-associated steatohepatitis or Mash, a liver disease.
Meanwhile, Eli Lilly’s Mounjaro has slashed the severity of sleep apnoea, a breathing disorder, by nearly two-thirds and cut heart failure outcomes in a separate trial.
Sattar, the University of Glasgow professor, says weight loss alone is a major driver of improvements across each of these disease areas.
“The weight loss attributes of the drugs are responsible for most of the benefits in multiple diseases, or are the predominant reason why these drugs affect multiple diseases,” he says.

As new and stronger drugs emerge, these effects are likely to increase. Novo Nordisk’s chief executive Lars Fruergaard Jørgensen said last month he was “very excited” about the potential of the company’s new CagriSema drug on diseases linked to obesity. Upcoming data is expected to show the drug delivers 25 per cent weight loss on average, an increase from the 22 per cent attributed to Mounjaro.
Danish professor Jens Juul Holst was among the first to identify the GLP-1 hormone in 1986, and sees the new crop of GLP-1s as similar in impact to bariatric surgery, a procedure to shrink stomach volume.
“The effects are becoming more and more clear, and now there’s no doubt that with the rate of weight loss you have, you also will have a real solution for steatosis [excess liver fat] in many individuals,” says Holst.
But weight loss alone does not explain the drugs’ benefits. Both Holst and Sattar pointed to a discontinued GLP-1 drug once marketed by GSK, albiglutide, that did not provide any weight loss in clinical trials, but led to cardiovascular benefits for patients.
There are GLP-1 receptors throughout the body and researchers believe the drugs could also have direct effects on organs and lead to health improvements other than through weight loss.
Recent trials exploring the effects of semaglutide in diabetics with chronic kidney disease showed a 24 per cent reduction in outcomes such as kidney failure for patients taking the drug.
“Weight loss may well be a contributor, but it would appear unlikely to be the whole explanation,” says Perkovic.

The drugs also lower inflammation in the body, which has been linked to better cardiovascular outcomes, while they could also be having “direct effects” on the kidneys, such as improving blood flow and reducing pressure in the kidney blood vessels, he adds.
Knowledge about the exact reasons for improved health outcomes is likely to increase as the drugs are more widely prescribed and drugmakers conduct larger studies.
“There will be a continuous flow of new results . . . which may or may not provide better explanations for exactly what goes on,” says Holst.
The wide-ranging action of GLP-1s has also triggered a search for still broader therapeutic frontiers.
One of the most intriguing is a late-stage trial of semaglutide in Alzheimer’s patients, with initial results expected in 2025. It follows early findings that patients with type 2 diabetes were less likely to develop Alzheimer’s disease if they were receiving GLP-1 medications.
Success would be “freaking nuts,” says one biotech investor, while noting that neurological disorders have for decades defied treatment. One pharmaceutical executive said scientists at his company were “sceptical” that the trial would bring positive results, partly because the drugs are not yet proven to cross the blood-brain barrier, a semi-permeable membrane that protects the brain.
Holst says “the most logical” way of explaining the potential benefits is through the lowering of inflammation and improvements in circulation. But it is still too soon to say whether GLP-1s offer a pioneering way to treat disorders afflicting growing numbers of people as societies age.
Studies exploring the drugs’ effects in combating Parkinson’s disease have also had mixed results. French scientists unveiled a study in April suggesting that lixisenatide — a GLP-1 diabetes drug — could slow the impact of the brain disorder, which causes balance and movement problems and other symptoms. Patients who took the medicine exhibited no further worsening of their motor symptoms after a one-year trial.
But exenatide, which had shown potential in an earlier trial of Parkinson’s patients, flopped in a new one reported in October. Olivier Rascol, co-author of the French study of lixisenatide, says the drug is quite similar in structure to exenatide, making it hard to explain why a trio of trials yielded such contrasting results.
“Nobody really has a clear explanation for [the] negative outcomes,” says Rascol, a Toulouse University Hospital professor and co-ordinator of the French NS-Park network for Parkinson’s research.

The Parkinson’s conundrum highlights wider gaps in knowledge about how GLP-1s work against brain disorders. Evidence for their effectiveness in treating addiction, for instance, has largely come from looking back at health records and observing that patients with addictions were less likely to overdose if they were using GLP-1s.
Sue Grigson, a scientist at Penn State University Hospital who has worked on small studies in opioid-use disorder, is among those exploring the links. She has observed a lower dopamine response when patients were using GLP-1s, indicating that the effect of the “feel-good” hormone is damped by the drugs.
Grigson said the drugs appear to have an effect on the circumventricular organs — the parts of the brain not protected by the blood-brain barrier — and this may influence brain function. But much remains unknown about the drugs’ impact on neurological conditions.
“This is still very much in infancy,” Rascol says, of the understanding of how GLP-1s affect the brain. “There will be more . . . experiments to better explore the real effects of these compounds.”
While scientists continue to explore their potential, pharmaceutical companies are already cashing in on GLP-1s’ broader health benefits.
Eli Lilly is seeking US regulatory approval for Mounjaro as a treatment for sleep apnoea, while Novo Nordisk has filed an application for Wegovy in chronic kidney disease.
“There’s a reason why companies have gone for these diseases where they think the weight loss plus or minus the direct effect may have a benefit,” says Sattar. “Guess what? The reality proves that to be the case.”
But large-scale trials are expensive and securing extra approvals could entrench the early movers’ grip on the GLP-1 market. “Novo and Lilly are going to cement their lead here,” said one investment banker.
Adam Steensberg, the chief executive of Danish biotech Zealand Pharma, which is developing an alternative weight loss product based on a different hormone, says “there is a duopoly already” in the GLP-1 market.
Combining different drugs could be a way of expanding the effects of GLP-1s further still. Doctors such as Perkovic envisage a possible future treatment regime for chronic kidney disease patients that combines existing drugs with GLP-1s to drastically reduce the risks of organ failure.
But the upfront costs of GLP-1s are likely to hold back the wide-ranging access needed if the treatments are to tackle cardiometabolic diseases across the world.
The US list price of Wegovy is $1,349 for a one-month course. The cost of preventing one major adverse cardiovascular event, such as a heart attack or stroke, is likely to be almost $1.3mn, Airfinity predicts. This estimate assumes such an outcome would on average require 67 people to be treated for over three years, with the drugs available at a large discount to the list price.
Side-effects including nausea, diarrhoea and fatigue also remain a problem for some users, a challenge drugmakers and physicians are attempting to address. BlueHealth Intelligence, a health insurer in the US, found that 30 per cent of GLP-1 users in the US were discontinuing their usage within a month, with poorer patients more likely to stop taking their drugs.
Glasgow University’s Sattar sees a “distant future” where lower-priced GLP-1s can be given to patients orally at the onset of conditions such as diabetes, tackling both obesity and other negative consequences in a way that doesn’t “bankrupt the health system”.
While progress is being made on cardiometabolic conditions, Ricks’ comments are the first sign that pharmaceutical companies will undertake clinical trials in areas such as addiction. Even if scientists establish a link, the road to an approval for use here is long.
The head of the US National Institute on Drug Abuse, Nora Volkow, points out that treating addiction may not be a commercial priority for pharmaceutical groups. GLP-1s are already generating big sales for Novo Nordisk and Eli Lilly, but many obese patients are yet to receive them — so there is still a huge target market.
“It may be hard, if the pharmaceutical industry says ‘why should I care? I make much more money prescribing these things for obesity’,” Volkow says.
Additional reporting by Oliver Barnes in New York